Since the year 2000, our Research Department has been actively involved in studies investigating Artificial Iris. We are thrilled that this device has been approved by the FDA! Click here to read more about this approval.
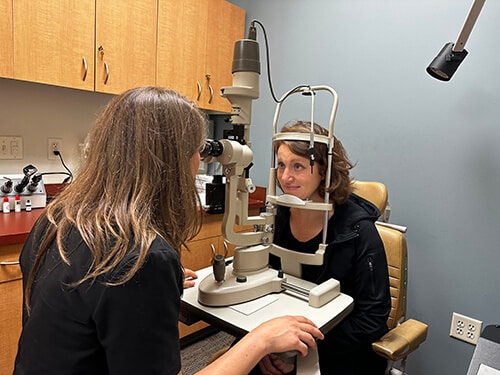
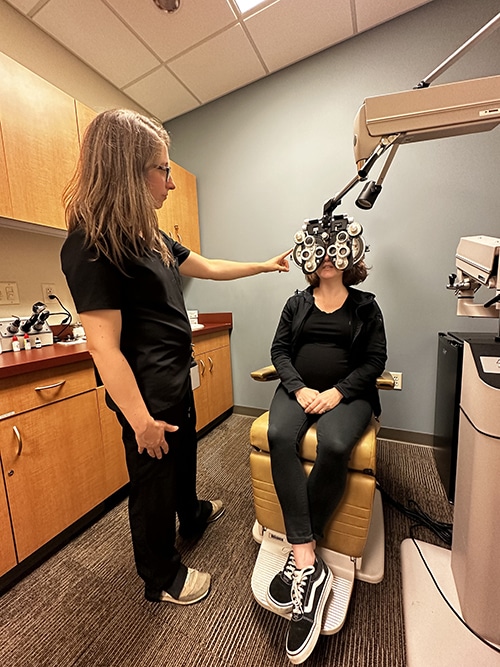
Minnesota Eye Consultants’ Clinical Research Department was created to ensure high-quality pre-clinical and clinical trials in vision research. Clinical research is an important component of our practice because it extends new technologies to patients and increases the overall standard of vision care. Our physicians, surgeons, and researchers have participated in vision research studies for years and are among a select group of individuals whose contributions led to many of the refractive surgery technologies in use today.
Research study success begins with a dedicated team of researchers and medical personnel who have years of experience in clinical trials, consulting, and management. The research team is comprised of Ophthalmologists, Doctor of Optometry, fellow physicians, a clinical research manager, clinical research coordinators, and technicians. Research activities are conducted within our metro-area clinics.
Major areas of investigation include, refractive surgery, complex case management, cataract surgery, natural lens replacement surgery, glaucoma management, corneal transplantation, iris reconstruction, surgical instrumentation, and drug therapies.
We are currently engaged in the following studies and are accepting enrollment of participants:
Director of Research: Dr. Sherman W. Reeves, MD, M.P.H.
Clinical Research Manager: Allison Dreyer
Clinical Research Coordinators: Kristen Dew, Ise Mondlicht, Meg Mack
Telephone: (952) 888-5800
Email: [email protected]
For more information, please contact the Minnesota Eye Consultants’ Clinical Research Department.
Bloomington Office
9801 Dupont Ave S, Suite 200,
Bloomington, MN 55431
Minnetonka Office
10709 Wayzata Blvd.
Minnetonka, MN 55305
Our Research Study Registry is a completely voluntary and secure database created and maintained by our research department. It allows our research staff to contact you about our current studies if we have one that appears to match your interests. These studies will have been previously reviewed and approved by an Institutional Review Board to evaluate the risks and benefits of each study.
Research Study Registry is not a research project – it is a registry of information about individuals who are interested in research and willing to be contacted about participation in research studies. If we contact you about a study, you are not required to participate in or volunteer for the study. If we provide you with information about an available study, it does not mean that Minnesota Eye Consultants is recommending that you participate in or consider participating in that study. And, you can always remove yourself from the Study Registry by emailing [email protected].
Minnesota Eye Consultants is proud to offer patients convenient access to eye care across the Twin Cities. We have 5 locations, each with an onsite ambulatory surgery center (ASC).